TMTPOST -- The global biotech sector was jolted on September 11 as reports emerged that the Trump administration was considering new sanctions targeting Chinese innovative drugmakers. The Hang Seng Biotech Index in Hong Kong fell 7% at the open, sending shares of leading companies such as BeiGene and CSPC Pharmaceutical Group sharply lower. Investors reacted swiftly, concerned that potential U.S. restrictions could undermine Chinese firms’ access to critical overseas markets.
However, the shock was short-lived. Less than 48 hours later, the National Medical Products Administration (NMPA) announced a major policy adjustment that immediately bolstered market confidence: the review and approval period for clinical trial applications of innovative drugs would be shortened to 30 working days—nearly halving the previous timeline.
U.S. Moves Target Chinese Biopharma Expansion
The United States has been intensifying scrutiny on Chinese innovative drugs entering the global market. By strengthening Committee on Foreign Investment in the United States (CFIUS) reviews and raising regulatory costs for FDA data submissions, the U.S. is aiming to restrict the core revenue streams of Chinese pharmaceutical companies abroad.
A report released by The New York Times recently indicated that the Trump administration’s draft executive order contains two key provisions directly affecting the critical stage of business development (BD) licensing. The first requires mandatory CFIUS review for U.S. acquisitions of rights to Chinese drugs under development, echoing the 2022 case when WuXi AppTec’s acquisition of U.S.-based CDMO Snapdragon was blocked. Analysts say this signals a potential systematic policy rather than isolated incidents.
China’s innovative drug sector is deeply entwined with the U.S. market. According to Industrial Securities, Chinese firms completed 83 license-out deals by the end of August 2025, a 57% increase year-on-year, with total disclosed deal value rising 185% to $84.5 billion. Any additional regulatory hurdles could extend deal timelines, raise costs, and weaken the bargaining power of Chinese drugmakers in international transactions.
The second provision targets overseas expansion by requiring the FDA to conduct more detailed reviews of Chinese clinical data and imposing higher fees on submissions. Historically, the success rate of Chinese clinical trials progressing from Phase I to FDA approval has been just 1.7%, highlighting the challenge of U.S. market entry. Currently, only BeiGene’s tislelizumab (TEVIMBRA®) and Junshi Biosciences’ toripalimab (LOQTORZI™) have secured FDA approval. Hengrui Medicine’s dual antibody therapy faced a forced resubmission, demonstrating the rigor of the U.S. regulatory process.
Despite the aggressive posture, the proposed executive order has faced internal U.S. pushback. MNCs seeking cost-effective innovation pipelines have increasingly partnered with Chinese companies. White House spokesperson Kush Desai noted that the administration is “not actively considering the draft.” Analysts argue enforcement is unlikely, given lobbying from multinational firms, practical challenges, and medical ethics considerations.
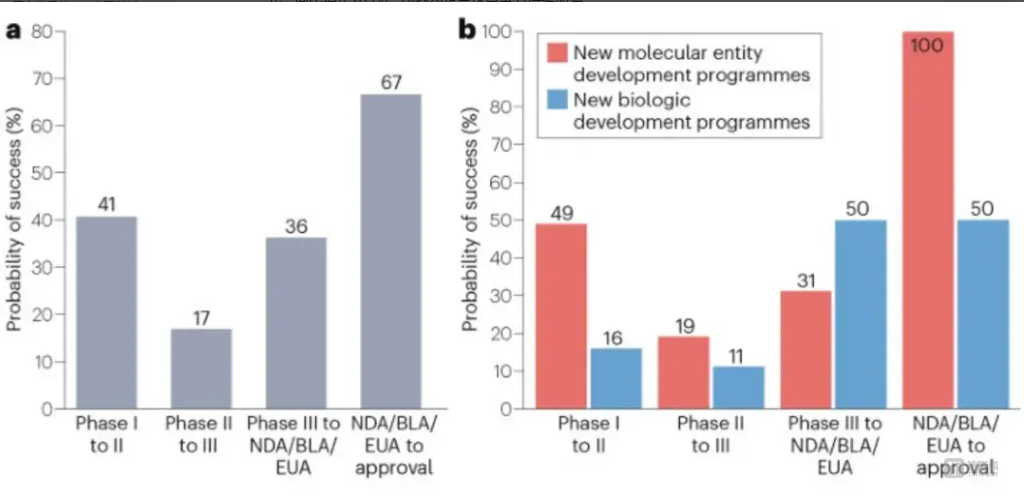
Success Rates of Chinese Innovative Drugs in U.S. Clinical Trials: a. Success rates by clinical phase; b. Success rates by drug type in each phase
Amid U.S. pressures, the NMPA’s new fast-track policy is a stabilizing force. Eligible Investigational New Drug (IND) applications can now be reviewed in just 30 working days, down from 60, covering traditional Chinese medicine, chemical drugs, and biologics meeting specific criteria.

This accelerated review compresses the R&D timeline, allowing companies to advance clinical trials more rapidly and iterate products faster. In an industry where the “three tens” rule—10 years, $1 billion, 10% success rate—prevails, speed is critical. Faster domestic reviews enhance China’s appeal in the global R&D network, encourage early-stage multinational collaboration, and strengthen bargaining power in licensing and financing negotiations.
In 2025, NMPA data shows 43 domestic innovative drugs approved in the first half of the year—a 59% increase year-on-year—with homegrown drugs accounting for 93% of approvals. China now represents more than 20% of global new drug development, ranking second worldwide, signaling rapid acceleration in innovation and commercialization.
The government has also implemented policies covering the entire drug lifecycle. The National Healthcare Security Administration’s “Several Measures to Support the High-Quality Development of Innovative Drugs” established a comprehensive framework for R&D support, market access, and insurance reimbursement. Measures such as commercial insurance coverage, improved hospital channels, and the “three exclusions” policy ensure innovative drugs are financially and clinically viable in the domestic market.
Contract Research Organizations (CROs) benefit as well, with advanced trial management systems, higher data quality control, and abundant site resources enabling faster clinical trials. This consolidation ensures that top CROs capture more clinical resources, while simplified insurance renewal approaches moderate price reductions and expand profit margins for truly innovative therapies.
China’s strategy emphasizes both domestic efficiency and global engagement. By compressing review cycles and providing robust insurance support, Chinese companies can build technological sovereignty while maintaining international partnerships. Diversified, compliant global networks mitigate risk from unilateral sanctions, positioning China’s innovative drug industry to compete on speed, quality, and efficiency in the global market.
As the United States debates restrictions and the NMPA accelerates domestic approvals, China’s biotech sector is rapidly adapting to a dual-track approach: securing rapid domestic development while strategically engaging with international markets. Analysts say this combination of regulatory efficiency, policy support, and global collaboration could cement China’s position as a leading hub for innovative pharmaceuticals.
更多精彩内容,关注钛媒体微信号 (ID:taimeiti),或者下载钛媒体 App